PFO Occluder
Occluder for Patent Foramen Ovale
Occlutech | PFO Occluder / Occluder for Patent Foramen Ovale
Occlutech | PFO Occluder / Occluder for Patent Foramen Ovale
Occlutech | PFO Occluder / Occluder for Patent Foramen Ovale
Occlutech | PFO Occluder / Occluder for Patent Foramen Ovale
Occlutech | PFO Occluder / Occluder for Patent Foramen Ovale
Description
The Flex II PFO Occluder allows physicians to simplify implantation and achieve cutting-edge results¹ through rapid training. Over 33,000 PFO occluders have been implanted in more than 70 countries worldwide with record effectiveness and safety².
Advantages
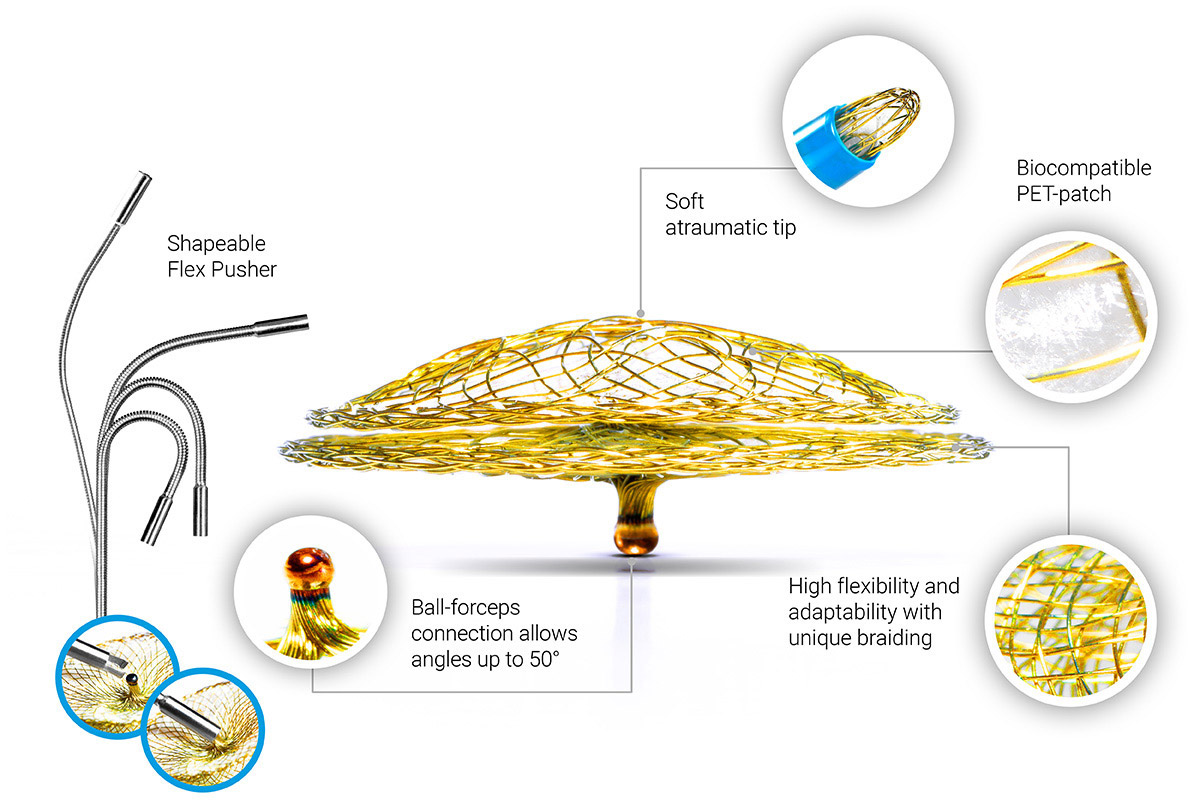
- The unique spherical connection between the pushrod and the occluder ensures a secure fixation while freely adapting to the anatomy. Once positioned, the occluder is easily and quickly deployed.
- The biocompatible handmade PET patch allows immediate verification of occlusion using ultrasound and X-ray imaging.
- Nitinol coated with titanium oxide demonstrates superior biocompatibility³ and minimal nickel release⁴.
- Optimized weaving: the absence of a hub and reduced material on the left atrial disc ensure better tissue integration.
Excellent Product Features
Links to Content
- Percutaneous Closure of the Patent Foramen Ovale with Occlutech: Safety and Efficacy Registry (OPPOSE), multicenter registry, UK.
- 2018_Snijder Closure of PFO in 250 patients and 1300 patient-years of follow-up using the Occlutech Figulla device.
- Castleman L.S., et al. Biocompatibility of Nitinol Alloy as Implant Material. Journal of Biomedical Materials Research | 97 6; 10: 695.
- Electrochemical Characteristics of Nitinol Occluder, Institute of Natural and Medical Sciences, University of Tübingen, mNI <0.014 μg / (cm * day).